Properties of Petroleum
Petroleum is not a single
substance. There are hundreds of different crude oils with a wide range of
physical and chemical properties; properties vary with location, depth, and age
of the oil field. Informally, crudes are named for their source and some key
characteristics; examples include Brent, West Texas Sour, West Hackberry Sweet,
and Arabian Heavy.
Formally, crudes are defined by a
crude assay, as described in a subsequent section. The “sweet” and “sour”
deserve comment. In the old days, prospectors characterized crude oil by
tasting it. Sour crudes have more sulfur, which gives them a tart taste.
Density, Distillation, and Elemental Composition
As produced, crudes contain
varying amounts of dissolved gas, water, inorganic salts, and dirt. After these
are removed, what remains is an exceedingly complex mixture of chemicals,
mostly organic hydrocarbons containing nothing but hydrogen and carbon. The
other organic molecules contain hetero-atoms—sulfur, oxy- gen, and nitrogen,
and/or trace elements (Ni, V, Fe, Cu, Hg, As, etc.). Processing costs are
higher for crudes with high density and large amounts of sulfur, nitrogen, and
trace contaminants.
To illustrate how widely crude
properties vary, Table 5 presents the density, sulfur, and nitrogen content of
21 example oils. The data from Table 5 are presented graphically in Fig. 10. Both sulfur and nitrogen correlate
inversely with API gravity, but for this particular collection, the
correlations are rough, especially for sulfur. Sulfur contents range from 0.03
wt% for Tapis to 5.3 wt% for Boscan, and nitrogen contents range from nil for
Tapis to 0.81 wt% for California Beta. Specific gravities range from 0.798 for
Tapis to 1.014 for Athabasca. By definition, Athabasca is “extra heavy oil,”
because its specific gravity is >1.0.
In other words, it sinks in water.
For “heavy oil,” the specific gravity falls between 1.0 and 0.934. The dynamic viscosities
of heavy oils range from about 5000 to 10,000 centipoise (cP).
Table 5 Density, sulfur, and nitrogen content of 21 crude oils
Fig. 10 Sulfur and nitrogen versus API gravity for selected crude oils
Distillation yields are an
exceptionally important property of petroleum, because they indicate relative
amounts of low-boiling fractions—naphtha (which can become gasoline), kerosene (which
can become jet fuel), and gas oil (which can become diesel).
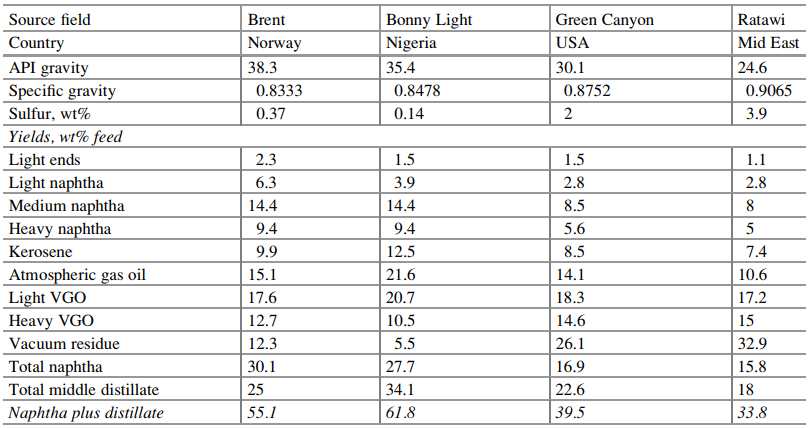
Table 6 Distillation yields for four selected crude oils
Markets are limited for direct use
of higher boiling fractions—atmospheric residue, vacuum gas oils, and vacuum
residue-so there is a large incentive to convert them into lighter products
with greater value. Table 6 shows distillation data for four common crudes.
Brent contains twice as much naphtha as Ratawi, and its vacuum residue content
is 60% lower. Of the four, Bonny Light yields the most middle distillate and
the least vacuum residue.
Distillation cutpoints for Table 6
are as follows:
Molecular Composition
More than any other element,
carbon binds to itself to form straight chains, branched chains, rings, and
complex three-dimensional structures. The most complex molecules are
biological—proteins, carbohydrates, fats, and nucleic acids. This is
significant, because petroleum was formed from the remains of ancient
microorganisms— primarily plankton and algae. As they aged in sediments under
elevated temperature and pressure, these biomolecules lost olefinic and hetero-
atom functional groups, leaving behind hydrocarbon skeletons.
Petroleum molecules can be
categorized as saturated, aromatic, and polar compounds, or as paraffins,
olefins, naphthenes, aromatics, polynaphthenes, polyaromatics, naphthenoaromatics,
and heteroatom compounds. Saturated hydrocarbons can be acyclic paraffins
(alkanes) or cyclic paraffins (naphthenes).
Olefins are very rare in natural
petroleum. They are mainly products from thermal cracking in refineries.
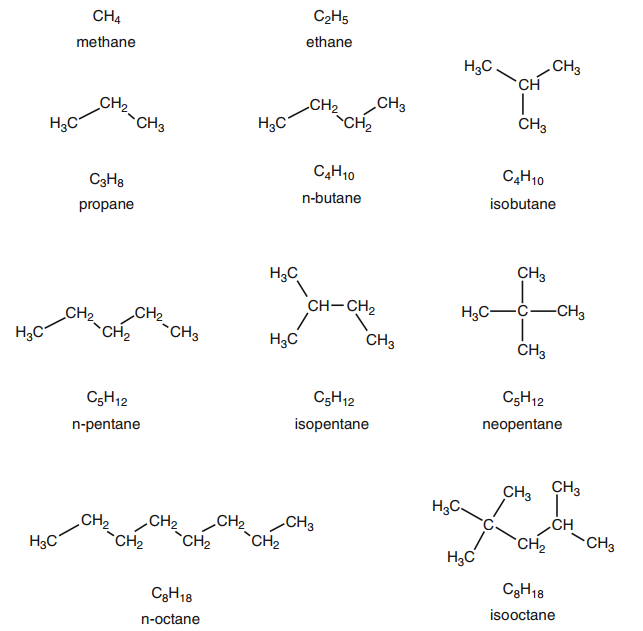
Fig. 11 Structures of some simple paraffins
Paraffins
Paraffins have a general formula of
CnH2n+2 . The simplest
paraffin is methane with a single carbon atom. Methane is the major component of
natural gas. The next member in the alkane family is ethane with two carbon
atoms. After that comes propane, with three carbon atoms. When the carbon
number reaches 4, isomers are possible. Isomers are chemical compounds with the
same molecular formula but different structures.
Normal paraffins are unbranched. No
carbon atom is connected to more than two other carbon atoms. In isoparaffins,
at least one carbon atom is connected to three or four other carbon atoms.
Carbon atoms connected to only one other carbon, such as the end-of-chain
carbons in n-paraffins, are called primary (1◦). Carbon atoms connected to two other carbons are called secondary (2◦), those connected to three
other carbons are called tertiary (3◦), and those connected to four other carbons are called (quarternary (4◦.
For example, C4H10 includes normal
butane (n-C4), in which all carbon atoms are primary or secondary, and
isobutane (methyl propane or i-C4), in which the central carbon atom is
tertiary. C5H12 can have three isomers, normal pentane, isopentane (2-methyl butane),
and neopentane (2,2-dimethyl propane), as shown in Fig. 11. The central carbon
in neopentane is quarternary.
The isooctane in the figure is one
of several isooctanes. Its official name is 2,2,4-trimethylpentane. This
molecule serves as a standard for gasoline combustion performance in
spark-ignition engines. By definition, its octane number ¼ 100.
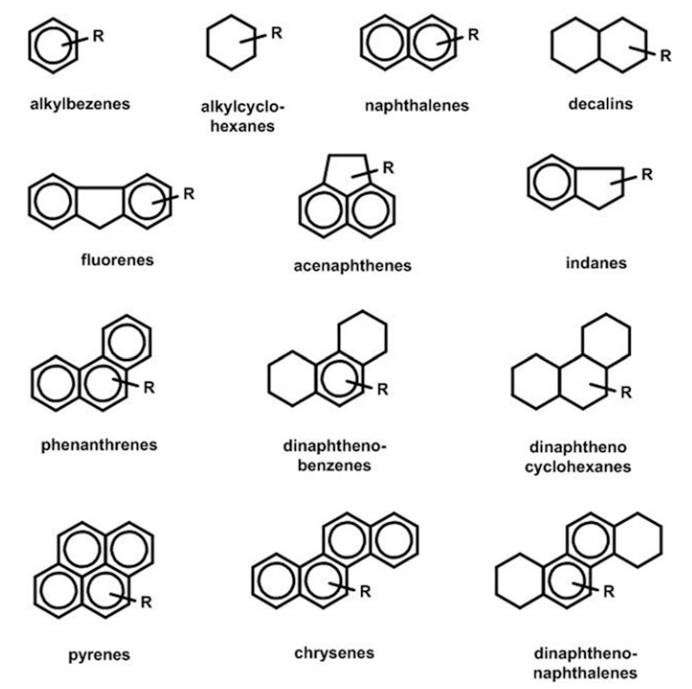
Hydrocarbon Ring Compounds (Naphthenes and Aromatics)
Figure 12 shows examples of
hydrocarbon ring compounds found in petroleum. In the figure, —R groups represent
alkyl chains.
Fig. 12 Example hydrocarbon ring compounds
Naphthenes are cyclic paraffins
with the gen- eral chemical formula CnH2n
. Naphthene rings can comprise 5-carbon atoms (cyclopentanes) or
6-carbon atoms (cyclohexanes). The rings generally contain paraffin side chains
with either normal or iso-structures. Decalins are dinap- hthenes with two
fused rings.
Aromatics contain unsaturated
rings. Monoaromatics have the
general formula CnH2n-6 ; the
ring contains three alternating (conjugated) double bonds, in which the
electrons are delocalized. The delocalization provides reso- nance
stabilization energy, which gives the rings stability. The simplest
monoaromatic is benzene. Like naphthenes, most aromatic rings are attached to
alkyl groups. Polyaromatics con- tain two or more rings; usually the rings are
condensed.
Naphthenoaromatics contain both
aromatic and naphthene rings. Usually the rings are fused. They are found
naturally in naphthenic crudes, and they generated by partial saturation of
polyaromatics in certain refining processes.
Heteroatom Compounds
Heteroatom compounds contain
sulfur, nitrogen, oxygen, and trace elements. Examples are shown in Fig. 13.
Sulfur is found primarily as H2S,
mercaptans, sulfides, disulfides, thiophenes, benzothiophenes, and
polybenzothiophenes. It also is found in ring compounds containing other
heteroatoms. Azathiophenes, for example, contain both nitro- gen and sulfur.
Fig. 13 Example heteroatom compounds
Nitrogen is present primarily
pyrroles, pyridines, quinolines, indoles, and carbazoles. Amides and oxazoles
contain both nitrogen and oxygen. Amines are not found in raw crudes.
Oxygen compounds include
naphthenic acids, carboxylic acids, phenols, cresols, and furans.
Trace elements such as Ni and V
tend to be incorporated into porphyrins, in which they are chelated by the
nitrogen atoms in the porphine ring. Ca- and Fe-containing porphyrins also have
been found. Arsenic and mercury are present as alkyl arsenes and alkyl mercury
compounds.
Other heteroatom compounds are
introduced during production and/or transportation. Iron naphthenates are
generated by naphthenic acid corrosion of steel, and organosilicon compounds
are added as flow improvers.
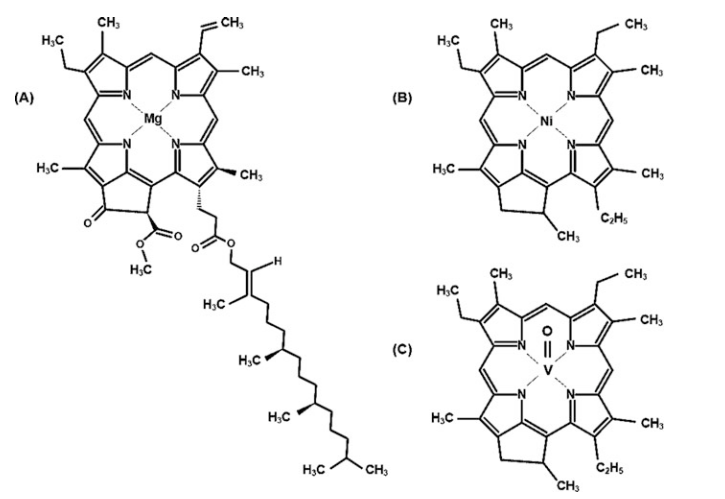
The discovery of porphyrins in
petroleum added weight to the theory that petroleum came from living organisms.
Figure 14 compares the
structure of chlorophyll A with
the structure of Ni-containing porphyrin and a V-containing porphyrin. Removal
of the side chains, oxygen atoms, and double bonds from the chlorophyll
structure, coupled with the replacement of the Mg by either Ni or VO(O),
generates the porphyrins.
Continuity Principle
The continuity principle [36]
states that the properties of the molecules in homologous series vary
monotonically. A homologous series is a group of compounds with the same
essential structure, which vary by a single parameter—such as the numbers of –H2C–
units in alkyl chains—or the number of condensed rings in polynaphthenes or
polyaromatics.
Figure 15 illustrates the continuity
principle for atmospheric equivalent boiling point (AEBP). The figure is grossly
simplified, but for carbon numbers less than 35, it does correspond with
reality. The indicated boiling points are for pure compounds. In mixtures, the
boiling points are shifted by molecular interactions. Molecular interactions
are greatest for polar compounds, such as those with hetero atoms.
Fig. 14 Comparison of chlorophyll A with a Ni-containing porphyrin (B) and a vanadium-containing porphyrin (C)
Fig. 15 Illustration of the continuity principle. Carbon number and atmospheric equivalent boiling points for different compounds
Boiling points are crucial,
because fractional distillation is the primary means by which petro- leum is
separated into useful products. Boiling ranges for typical cuts—naphtha,
kerosene (including jet fuel), AGO (atmospheric gas oil, including diesel fuel),
vacuum gas oils, and residue—appear at the top of the figure. In practice, due
to imperfect separation in commercial distillation towers, the fractions
overlap.
Curve A is for normal paraffins.
Branched isomers with the same carbon number (not shown) boil at lower
temperatures. Fully saturated polyring compounds fall on Curve B. Fully unsaturated
poly aromatics fall on Curve C. Curve D shows how adding alkyl groups to pyrene
makes Curve D parallel to Curve A. Curves E, F, and G represent compounds
containing hetero atoms—sulfur, nitrogen, and oxygen.
Phenanthrene (C14H10), a
three-ring poly aro- matic compound, is found in the AGO boiling range. Adding
seven hydrogen molecules (14 hydrogen atoms) converts
phenanthrene into perhydrophenanthrene (C14H10), shifting it into the kerosene
cut.
Crude Assay
Analyses of crude oils are
summarized in crude assays. An example crude assay report template is presented
in Fig. 16. There is no industry- standard testing grid—each company has its
own. Distillation properties determine straight-run yields of key fractions.
Elemental composition and total
acid number (TAN) indicates how expensive it will be to process the crude in a
refinery. Viscosity, freeze point, and pour point reflect how a fraction will
perform in a cold environment. Density, aniline point, and K-factor, along with paraffins, naphthenes, and aromatics, describe molecular composition, which determines how the fraction will behave in a
refinery.
Cetane number and diesel number
are important properties of diesel fuel. The last six properties—heptane
asphaltenes, microcarbon residue, Ramsbottom carbon, V, Ni, and Fe—determine to
a large extent the cost of upgrading the residue.
References
28. List of Largest
Companies by Revenue.
https://en.
wikipedia.org/wiki/List_of_largest_companies_by_ revenue. Accessed 2 Jan 2016
29. http://www.forbes.com/global2000/#. Accessed 2 Jan 2016
30. “Peak Oil,” https://en.wikipedia.org/wiki/Peak_oil. Accessed
5 Dec 2016
31. DM Fenton, H
Hennig, and RL
Richardson, The chemistry of
shale oil and its refined products. Symposium on oil shale, Tar
Sands and Related
Materials, American Chemical
Society Annual Meeting,
San Francisco (August 1980)
32. Mims N, Bell M, Doig S, (2009) Assessing the Elec- tric Productivity Gap and the U.S.
Efficiency Oppor- tunity. Rocky Mountain Institute, Snowmass, Colorado
33. WebElements: the periodic table on the web. http:// www.webelements.com/helium/. Accessed
1 Nov 2011
34. Energy Density. http://en.wikipedia.org/wiki/Energy_ density. Accessed
November 2011.
35. China’s coal emissions responsible for ‘quarter of a million
premature deaths’. http://www.theguardian. com/environment/2013/dec/12/china-coal-emissions- smog-deaths. Accessed
5 Jan 2015
36. Altgelt KH, Boduszynski MM (1994) Composition and analysis of heavy petroleum fractions. Marcel Dekker,
New York







Comments
Post a Comment