Types of chemical bonds
1. Ionic bond
2. Covalent bond
3. Pure covalent bond
4. Polar covalent bond
5. Coordinate bond
6. Metallic bond
7. Hydrogen bonding
Chemical bonds
Chemical bonds are the phenomenon
of the presence of atoms coherent together in molecule or crystal. The material
atoms are bonded together by chemical bonds, the type and strength of the
chemical bond depends on the electronic configuration of the constituent atoms
that form bond.
There are several types of chemical
bonds can form between the atoms of different elements, which are:
Ionic bond
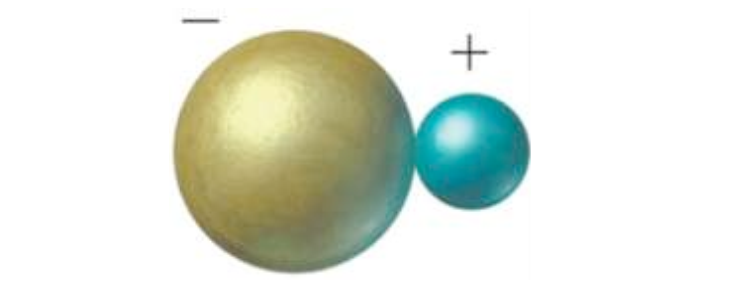
Ionic bonds emerge between a
metallic element and a non-metallic element through loss and gain electrons. It
results from the interaction of two atoms, one of which have High electronegativity (such as atoms of halogen group elements) and others have low
electronegativity (e.g., alkaline earth metals group and alkaline metals group
elements).
In this case, the valence electron
will move completely from the atom of the low electronegative element into
the atom of the high electronegative element, and then we have two ions, the
first is positive charge ions as a result of the loss of an valence electron ,
the second is negative ion as a result of the reception of this electron [Figure
1].
Figure 1: Ionic
bond of NaCl.
These different charged ions are
then bound as a result of the electrostatic attraction to form the complex and
form neutral crystal lattice [Figure 2] like; sodium chloride NaCl, potassium
chloride, KCl, magnesium chloride MgCl2 and potassium fluoride KF and calcium
chloride CaCl2 , as well as all hydrides of alkaline metals group elements and
alkaline earth metals group elements, such as sodium hydride NaH.
Figure 2: Crystalline structure of sodium chloride.
The most important properties of
ionic compounds are:
1. It exists in form of a crystalline structures, a
regular geomet- ric arrangement of negative and positive ions.
2. It has very high melting and boiling point, to
overcome the attraction forces between the negative and positive ions and to
break the crystalline structures.
3. Inability of conductive electrical in solid state due
to ions connection and their inability to move within the crystalline
structures while becomes conductive electrical, when melted or dissolved in
water (then the ions will be free to move in the melted and aqueous solution).
4. Soluble in polar solvents such as water and do not
dissolve in non-polar organic solvents such as gasoline or ether.
Covalent bond
Covalent bond is formed when the
valence electron is difficult to transmit a complete transition from one atom
to another, in this case the pair is formed from electronic contribution or
participation of both atoms. Charges are not shown on atoms, covalent bonds
often occur between non- metals.
The difference in the
electronegativity value (see electronegativity values for some elements in
Table 1) plays an important role in the formation of covalent bonds. It can
lead to two types of covalent bonds:
A- Pure covalent bond:
This bond emerge between two atom
of non-mitallic elements, where they are similar in electronegativity, or between
two elements atoms that are similar in electronegativity, where the difference
in electronegativity is zero for the two case. The pair of electrons will spend
equal time in the acquisition of both atoms [Figure 3].
Figure 3: Pure
covalent bond.
An example of this type of
covalent bond is the bond in the nitrogen molecule N2, the chlorine molecule
Cl2 and the oxygen molecule O2 and in the fluorine molecule F2.
B- Polar covalent bond:
This bond emerge between two
elements atom where they are similer electronegativity but in this case the
difference must be greater then zero and less than 1.7 to participation with
one or more electrons pair [Figure 4].
Figure 4: Polar
covalent bond.
Examples of this type of covalent
bonds found in molecules H2O, and ammonia NH3 of water H2O, ammonia NH3,
Hydrogen fluoride HF, aluminum chloride AlCl3, and brominde HBr, In this case
one of the atoms carry partial negative change (negative delta δ-) and the second atom carry partial positive change (positive delta δ+).
Table (1)
Electronegative values of some elements of the periodic table.
There are many types of covalent
bonds that differ in number of electronic couplings bonding between atoms.
most common covalent bonds is a
single bond, which share only one electronic pair, such as molecule F2.
Single covalent
bond.
When participating in two
electronic pairs, they are called double covalent bonds, In the case of
participation in three electronic pairs makes it a triple covalent bond. An
example of double bond is what we find in the oxygen molecule O2. An example of
triple bond is what we find in the nitrogen molecule N2.
Double covalent
bond.
Triple covalent
bond.
Covalent bonds compounds are
characterized by the following:
1. Low melting and boiling point, so do not need high
heat energy, because the forces of attraction between their molecules are weak.
2. Do not conducted electric current because they dont
form negative or positive ions in their melts or aqueous solutions.
3. Mostly don’t dissolve in polar solvents as water while
dis- solve in organic solvents such as ether and benzene.
Coordinate bond
Coordinate bond is formed when one
of the atoms provides a pair of electrons to another atom have the ability to
receive this electronic pair to form bond. Then this pair will be shared
between two atoms. The electron donor atom is the Lewis base and contains a
pair of free electron such as oxygen atom in a water molecule or a nitrogen
atom in the ammonia molecule.
The receiving atom is often a
transitional metals (Lewis acid) because they have empty orbitals of the type d
such as nickel or hydrogen atom ion.
We can say that coordinate bond Is
a special type of covalent bond, except that the source of the electron pair is
only from one atom, and the coordinate bond is longer and weaker than covalent
bond.
Example 1
Graphically illustrate the
emergence of the coordinate bond in the ammonium ion NH4 +
Solution
This ion consists of the binding
of ammonia NH3 to the hydrogen ion H+ in aqueous solution:
Note from the previous example
that the nitrogen atom has a pair of electrons not involved in the NH3 molecule
which can be involved, The hydrogen ion has an empty orbital that can receive
this pair.
When the ammonia molecule is close
enough to hydrogen an attraction occurs and the pair of electrons shared
between, as in covalent bond exactly and form NH4 + ion. This type of bond is
called coordinate bond and is referred to in the order of the Lewis symbol with
an small arrow rather than a line that represents a covalent bond.
Metallic bond
A chemical bond that happens
between the atoms of an element of metals, this bond is formed due to the metal
atoms possessing electrons in their outer shells contribute to the formation of
a crystal and these atoms has free electrons movement in this crystal.
When metal atoms are binding
together, they do not reach the electronic configuration of noble gases. Atoms
of metals such as sodium and potassium, they are easy to lose their equivalent
electrons and become positive ions because their electronegativity is low.
The strength of the metal bonds is
affected by several factors, the most important is the charge density which is
equal to the ion charge / ion size (proportional to the number of orbits),
where the ion charge is the charge that the metal gains after losing electrons
in the last orbit (+1,+2,+3).
Therefore, the force of the
metallic bond depends on the number of the electrons valence beam in the metal
atoms, the more electrons of the valence beam, the greater the coherence of the
metal to be more solid and higher in boiling point. Higher charge density on
the ion increased bond strength and as a result higher melting point obtained.
Many of the properties of natural
metals depends on the nature of this bond, electrical conductivity and thermal
conductivity of metals caused by the movement of free electrons between atoms.
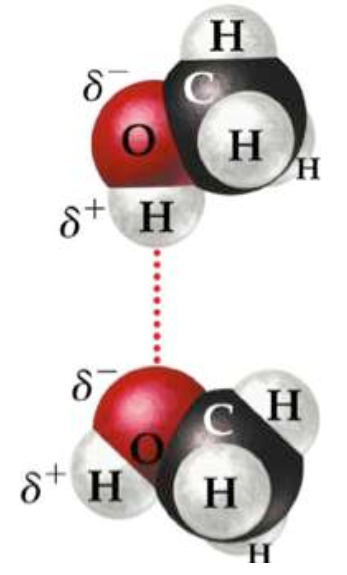
Hydrogen bonding
Hydrogen bonds arise due to the
attraction that occurs between the positive terminal (hydrogen atom) and the
negative terminal atom possess electronic pair or more. These three properties
are limited to three elements only are ; oxygen, fluorine and nitrogen atoms.
Therefore we find water, ammonia, hydrogen fluoride molecules and others are
agglomerated by effect of hydrogen bonds.
Hydrogen bond in
water molecule.
The hydrogen bond is the cause of
the high boiling point of water, the melting point of ice and the expansion of
the volume of frozen water that led to ice floats on the water. Hydrogen bond
is a weak physical bonding force between molecules and not a precise chemical
bond: therefore its strength is much lower than other.
Hydrogen bond.






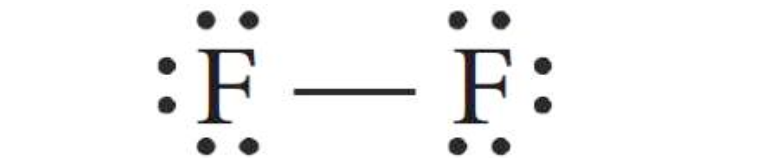
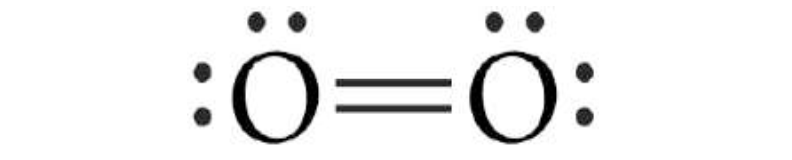
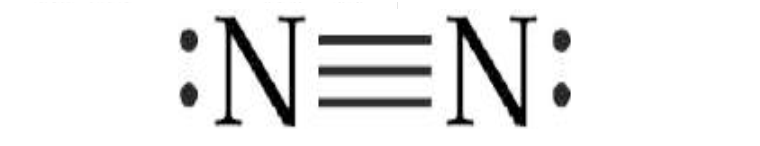


Comments
Post a Comment